Introduction
Multiple myeloma (MM) is a clonal plasma cell malignancy and is the second most common type of hematologic cancer worldwide. Treatment options for MM have evolved in the last two decades with the introduction of novel therapeutic agents such as immunomodulatory drugs (IMiDs), proteasome inhibitors (PI), anti-CD-38 monoclonal antibodies (anti-CD38 MoAb), and recently BCMA-directed therapies (BDT). The variable access globally, especially for novel agents, has been a concern. We aim to study the global availability of 14 chemoimmunotherapy options in addition to autologous stem cell transplant (ASCT) which is approved in the US (excluding BDT due to limited availability), to identify regions with restricted access and analyze the main barriers to access.
Methods
A global cohort of oncologists outside the USA who treat MM participated in an online survey to evaluate the patterns of accessibility of MM therapies worldwide. The survey, conducted in collaboration with the US Myeloma Innovations Research Collaborative (USMIRC), was sent electronically to 176 oncologists worldwide (outside the US) from June 18th, 2023, to July 17th, 2023. It included questions on demographics, access, and perceived barriers. The respondents chose a response for each therapy as being “easily accessible”, “moderately accessible,” “not readily accessible” or “no access”. Countries were then classified as having “adequate access” if 80-100% of respondents affirmed easy/moderately easy access, while <30% were considered “limited access”, 60-79% were “high-intermediate access” and 30-59% were “low-intermediate access”. We classified countries into low- and high-income (LIC and HIC) based on the United Nations (UN) World Economic Classifications based on per capita gross national income (GNI). Means were calculated for regions of interest. An interim analysis is reported.
Results
Demographics: Ninety-Five (54%) oncologists from 33 countries completed the survey. Most were from Asia (56%). Others were Europe (19%), South America (12%), Africa (7%), Oceania (3%), and North America (Canada and Mexico, excluding the US) (3%). Only 17% specialized in plasma cell disorders; 50.5% were affiliated with university academic centers; 18% with private or community practices; 12% with community academic centers; and 17% were hybrid. Public-funded centers represented 61.1%, while 76% were transplant centers.
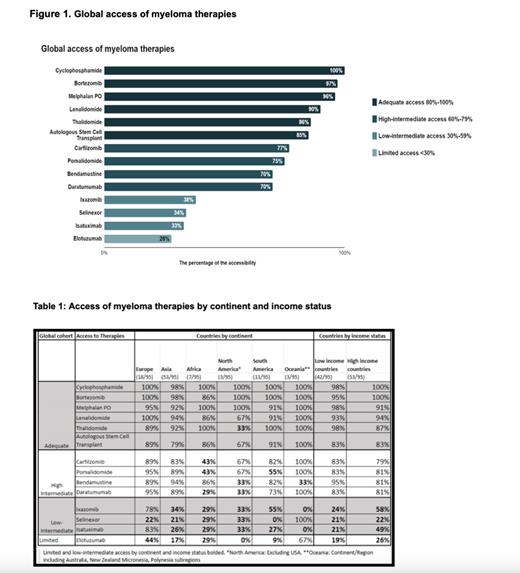
Global Patterns: Most countries had adequate access to chemotherapies, IMiDs, PIs, Anti-CD38, MoAbs, and ASCT (Figure 1). Notably, ixazomib, isatuximab, and selinexor had low intermediate access, while elotuzumab had limited access.
Access by Income: Most MM therapies were available in LIC and HIC. Of the therapies listed, 6 were adequately available in LIC and HIC: cyclophosphamide, lenalidomide, pomalidomide, daratumumab, bortezomib, and ASCT (Table 1). Elotuzumab and selinexor were limited in both LIC and HIC. Ixazomib and isatuximab were also limited in LIC but were low-intermediate in HIC.
Access by Continent: Out of the 14 therapies, Europe had adequate or high-intermediate access to most modalities (12), followed by Oceania and Asia (10), and South America (9). North America and Africa had the lowest number of adequate or high intermediate access therapies (7) (Table 1).
Barriers: The most common reason cited for limited access to MM therapies globally was the financial burden for the healthcare system, followed by limitations in local agency approval, inadequate chemotherapy suite resources, and inadequate staffing.
Conclusion
While most therapies were available, novel therapies such as isatuximab, ixazomib, selinexor, and elotuzumab were less readily accessible. Moreover, North America and Africa had the least access to therapies. This is likely because LIC and HIC have prioritized access to treatments with the best evidence of cost-effectiveness, while those with lower cost-effectiveness are generally limited. The financial burden on healthcare is a major limiting factor. USMIRC plans to investigate potential solutions and pursue global collaborative efforts to reduce disparities in therapies.
Disclosures
Popat:GSK: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; Janssen: Honoraria; BMS: Honoraria; Roche: Honoraria. Mahmoudjafari:Omeros: Speakers Bureau; Pfizer, Genentech, Inc., BMS, KITE, Sanofi, Janssen: Honoraria; Genentech, Inc.: Consultancy. Ahmed:Kite/Gilead: Consultancy, Research Funding; BMS: Other: Ad Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal